Emergence of a Deadly Coronavirus
FDA Says Pfizer-BioNTech Vaccine Is Safe, Effective
The Food and Drug Administration said the first Covid-19 vaccine being considered for U.S. distribution “met the prescribed success criteria” in a clinical study, paving the way for the agency to green-light distribution as early as this weekend.
An outside panel of scientific advisers will review the FDA report Thursday, along with a companion analysis from the vaccine’s manufacturers, Pfizer Inc. and German partner BioNTech SE. A favorable recommendation from the panel is expected to be followed within a few days by the FDA granting emergency authorization for the vaccine.
In its report Tuesday, the FDA noted that the two-dose vaccine provided benefits even after just the first injection—cutting the risk of getting Covid-19 by about half. The vaccine was found to be 95% effective after the second dose, three weeks later.
FDA scientists also found that the vaccine was effective in reducing the risk of confirmed severe disease after the first dose, an important finding as some health experts were concerned Covid-19 vaccines would protect against only mild to moderate disease.
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
ASPartOfMe
Veteran

Joined: 25 Aug 2013
Age: 67
Gender: Male
Posts: 36,777
Location: Long Island, New York
’Quite frankly shocking’: US COVID-19 deaths hit record levels as a vaccine appears days away
Virtually every state is reporting surges just as a vaccine appears days away from getting the go-ahead in the U.S.
“The epidemic in the U.S. is punishing. It’s widespread. It’s quite frankly shocking to see one to two persons a minute die in the U.S. — a country with a wonderful, strong health system, amazing technological capacities,” said Dr. Michael Ryan, the World Health Organization’s chief of emergencies.
The virus is blamed for more than 280,000 deaths and almost 15 million confirmed infections in the United States.
The WHO’s Ryan said that the U.S. is accounting for one-third of all world cases over the last several weeks and that the “brutal reality” is that holiday hugs are ill-advised.
In Georgia, the number of confirmed and suspected coronavirus infections has soared more than 70% in the past week, and hospitals are sounding alarms about their ability to absorb new COVID-19 patients.
Health experts warn that well after the vaccine arrives in the U.S., masks and social distancing will remain vital to bringing the coronavirus under control.
In Pennsylvania, overwhelmed hospitals may have to begin rationing care and turning away patients, Gov. Tom Wolf warned Monday, calling it a “dangerous, disturbing scenario.”
In Nevada, the number of people hospitalized with COVID-19 has more than tripled over the last month, as did the number of patients needing ventilators.
_________________
Professionally Identified and joined WP August 26, 2013
DSM 5: Autism Spectrum Disorder, DSM IV: Aspergers Moderate Severity
“My autism is not a superpower. It also isn’t some kind of god-forsaken, endless fountain of suffering inflicted on my family. It’s just part of who I am as a person”. - Sara Luterman
The Food and Drug Administration said the first Covid-19 vaccine being considered for U.S. distribution “met the prescribed success criteria” in a clinical study, paving the way for the agency to green-light distribution as early as this weekend.
An outside panel of scientific advisers will review the FDA report Thursday, along with a companion analysis from the vaccine’s manufacturers, Pfizer Inc. and German partner BioNTech SE. A favorable recommendation from the panel is expected to be followed within a few days by the FDA granting emergency authorization for the vaccine.
In its report Tuesday, the FDA noted that the two-dose vaccine provided benefits even after just the first injection—cutting the risk of getting Covid-19 by about half. The vaccine was found to be 95% effective after the second dose, three weeks later.
FDA scientists also found that the vaccine was effective in reducing the risk of confirmed severe disease after the first dose, an important finding as some health experts were concerned Covid-19 vaccines would protect against only mild to moderate disease.
Looks like the FDA did no testing?
_________________
Then a hero comes along, with the strength to carry on, and you cast your fears aside, and you know you can survive.
Be the hero of your life.
Biscuitman
Veteran

Joined: 11 Mar 2013
Age: 45
Gender: Male
Posts: 2,674
Location: Dunking jammy dodgers
The FDA is the approving body.
The U.S. Food and Drug Administration (FDA) is the regulatory authority that has oversight of the safety, effectiveness and quality of vaccines that are used in the United States.
Vaccines to prevent infectious diseases are given to millions of babies, children, adolescents and adults and it is critical that they are demonstrated to be safe and effective. Vaccines have prevented countless cases of disease and disability and have saved millions of lives. Ensuring the safety and effectiveness of vaccines is one of FDA’s top priorities.
FDA’s Center for Biologics Evaluation and Research (CBER) ensures that FDA’s rigorous scientific and regulatory processes are followed by those who pursue the development of vaccines. Vaccine development is a complex science. FDA’s scientific and regulatory advice to vaccine developers, as well as FDA’s evaluation to determine the safety and effectiveness of vaccines, are among the most robust in the world.
This link shows the FDA approval process - Vaccine Development – 101
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
U.K. regulators said Wednesday that people who have a "significant history'' of allergic reactions shouldn't receive the new Pfizer-BioNTech vaccine while they investigate two adverse reactions that occurred on the first day of the country's mass vaccination program.
"As is common with new vaccines the MHRA have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday,'' Powis said in a statement. "Both are recovering well."
UK investigates possible allergic reactions to Pfizer coronavirus shot
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
"Sputnik V" Coronavirus Vaccine
Last week, President Vladimir Putin ordered the launch of a “large-scale” COVID-19 immunization campaign, prioritizing doctors, teachers and other essential workers. Russia has said 100,000 people have already been vaccinated, though the vaccine has been widely criticized because it was approved over the summer after tested on only a few dozen people.
Last month, developers of the vaccine said interim analysis of trial data showed it was 91.4% effective. The conclusion was based on 39 infections among 18,794 study participants that received both doses of either the vaccine or a placebo.
Russian citizens have been warned to curb alcohol intake for two months after receiving the Sputnik V coronavirus vaccine, per news reports. Deputy Prime Minister Tatiana Golikova told TASS, the Russian news agency, that vaccination will take 42 days and citizens need to avoid alcohol and immunosuppressant drugs while following mitigation measures to avoid infection. In general, the medical community has long recognized that excessive drinking can weaken the immune system. “Clinicians have long observed an association between excessive alcohol consumption and adverse immune-related health effects such as susceptibility to pneumonia,” and more recently, respiratory distress related-syndromes, according to NIH-published research.
Source: After coronavirus vaccination, Russians warned to avoid alcohol for 2 months
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
Seven vaccine candidates are expected to pass FDA muster in the next months. By the end of March, we should have enough vaccine available to finish vaccinating health care workers, nursing home residents, and other high-risk candidates. By May, it is expected that 70% of the US population will be vaccinated, at least according to the head of the federal Operation Warp Speed program. That seems a bit overly optimistic given some recent polls stating only 50% of Americans would accept the vaccine once it receives FDA approval. Worse, as a Yahoo Finance-Harris Poll reports, nearly two-thirds of Americans have concerns about the fast-tracked COVID-19-vaccines.
Source: Vaccines Are Ready. Come And Get 'Em, Or Should We Institute A 'Pay-For-Jab' Program?
IMHO I would not put much stock in the polls. The decision will be driven out of fear. Fear of dying from COVID19 vs. fear of dying from the vaccine. Most individuals are afraid of being the first out of the gate. But as more and more people become vaccinated, a true picture of real or imaginary dangers will materialize.
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
Health authorities from the United Arab Emirates on Wednesday announced that a Chinese coronavirus vaccine had an 86% efficacy rate against infection in human trials. The UAE Ministry of Health and Prevention shared the findings through the state-run WAM news agency. Phase 3 trials from Sinopharm’s China National Biotec Group’s (CNBG) inactivated COVID-19 vaccine also revealed antibodies in 99% of participants, and 100% effectiveness against moderate to severe cases of COVID-19 disease.
Source: Chinese coronavirus vaccine 86% effective, UAE health leaders say
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
The FDA is the approving body.
The U.S. Food and Drug Administration (FDA) is the regulatory authority that has oversight of the safety, effectiveness and quality of vaccines that are used in the United States.
Vaccines to prevent infectious diseases are given to millions of babies, children, adolescents and adults and it is critical that they are demonstrated to be safe and effective. Vaccines have prevented countless cases of disease and disability and have saved millions of lives. Ensuring the safety and effectiveness of vaccines is one of FDA’s top priorities.
FDA’s Center for Biologics Evaluation and Research (CBER) ensures that FDA’s rigorous scientific and regulatory processes are followed by those who pursue the development of vaccines. Vaccine development is a complex science. FDA’s scientific and regulatory advice to vaccine developers, as well as FDA’s evaluation to determine the safety and effectiveness of vaccines, are among the most robust in the world.
This link shows the FDA approval process - Vaccine Development – 101
The FDA is doing no testing?
The earlier article came across phony, because it made it seem lke the FDA did clinical trials, when it apears like they did no testing at all.
_________________
Then a hero comes along, with the strength to carry on, and you cast your fears aside, and you know you can survive.
Be the hero of your life.
Health and Human Services Secretary Alex Azar said vaccinations could begin for “the most vulnerable” next week, and he expects 20 million people to be vaccinated before the end of the year. He said, pending FDA emergency use authorization, 50 million Americans could be vaccinated by the end of January, saying, “As soon as we have authorization from FDA, we plan to ship vaccines to our 64 public health jurisdictions within 24 hours.” General Gus Perna, who is in charge of vaccine distribution, did say there may only be 2.9 million shots in the arms of Americans in the first week. Vaccines would then be distributed on a rolling basis. The Food and Drug Administration will meet Thursday to review the Pfizer-BioNTech vaccine candidate for EUA, and preliminary documents did not flag any new concerns or safety issues regarding the vaccine after reviewing the submitted data.
The United Kingdom approved the vaccine and began inoculating citizens this week. Canada approved the Pfizer vaccine on Wednesday.
Source: Azar says vaccinations could begin next week, 20M people inoculated by end of year
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
Don’t know if this covid map has been posted.
https://covidactnow.org/?s=1416388
_________________
I am the dust that dances in the light. - Rumi
Here is a link to the FDA Briefing Document on the Pfizer-BioNTech COVID-19 Vaccine. It was posted on the Internet 3 minutes ago.
FDA Briefing DocumentPfizer-BioNTech COVID-19 Vaccine
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
First Vaccine Approved
A U.S. Food and Drug Administration advisory committee on Thursday voted to approve Pfizer and BioNTech’s emergency use authorization (EUA) application for its coronavirus vaccine candidate, the first COVID-19 jab to win such approval in the U.S. The vote was 17-4. One committee member abstained.
Health care workers and residents of long-term care facilities will be the first to receive the long-awaited coronavirus vaccine.
The agency found no specific safety concerns among subgroup analyses but did list several unknowns that will need to be investigated further, including duration of immunity, efficacy in certain high-risk populations, those previously infected, as well as effectiveness among asymptomatic infection, long-term effects of COVID-19 disease, mortality and transmission of SARS-CoV-2.
Fatigue, headache, muscle pain, chills, joint pain and fever were all listed as reported adverse reactions but were categorized as mild to moderate. Serious adverse events remained uncommon and “represented medical events that occur in the general population at similar frequency as observed in the study.”
Source: FDA committee votes to OK Pfizer's coronavirus vaccine for EUA
The FDA is expected to meet on December 17 and consider approval for the Moderna vaccine.
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
Aerosol Transmission
The German Working committee particulate matter (AAF) brings together experts from the fields of engineering, chemistry, physics, biology, meteorology and medicine, who are organized in the professional associations ProcessNet (DECHEMA/ VDI-GVC), Gesellschaft Deutscher Chemiker (GDCh) and VDI/DIN Commission Reinhaltung der Luft (KRdL). In its autumn meeting, the AAF discussed the role of aerosol particles in the spread of the SARS-CoV2 viruses and prepared a statement on this topic.
Aerosols and their spread play an essential role in the transmission of COVID-19. However, the risk of transmission could be significantly reduced if more could be done to reduce indoor airborne viruses. The Working committee particulate matter (AAF) has therefore issued an statement with concrete recommendations. These include window ventilation, exhaust ventilation, air purification systems and CO2 measuring devices for indoor areas such as classrooms or transportation, and the increased use of N95 and FFP2 masks. These countermeasures could help in the short term to better contain the corona pandemic, especially in winter, until vaccination is effective on a large scale. They could also help in the long term to better control infections such as seasonal flu or even other pandemics in the future.
Source: COVID-19 pandemic could be better tackled by reducing aerosol transmission
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."
Vaccines Effectiveness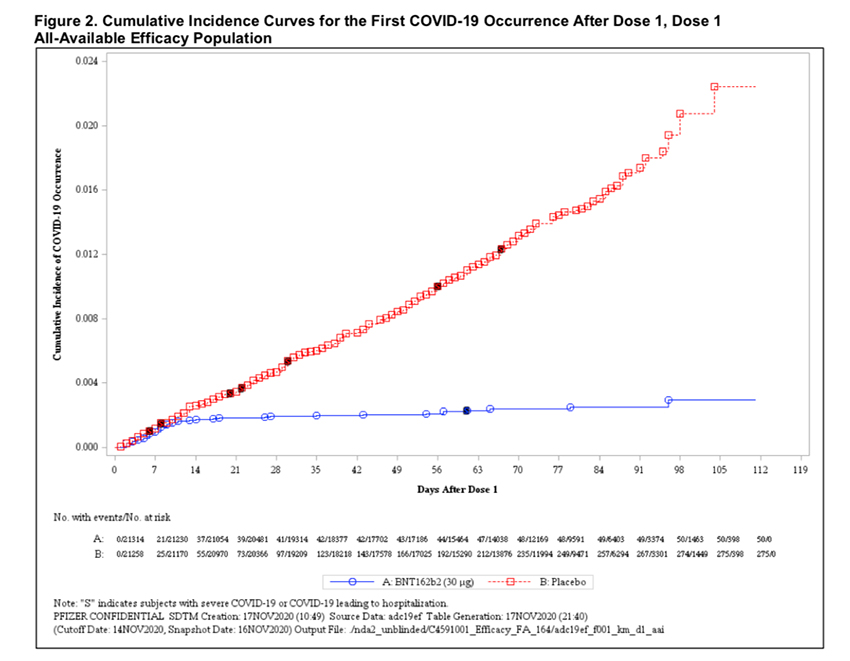
In the analysis of the data, the efficacy of the initial dose is upwards of 88%. The blue line reflects COVID-19 infections in the vaccine arm; the continuously rising red that of the placebo. But as researchers write, the rapid onset of protection means that “this is potentially important as we could vaccinate more people in a hot-spot and potentially delay the second dose.” Could it be that an end is coming into view?
Source: Every Picture Tells A Story: How Effective Is The Pfizer Vaccine?
_________________
Author of Practical Preparations for a Coronavirus Pandemic.
A very unique plan. As Dr. Paul Thompson wrote, "This is the very best paper on the virus I have ever seen."





